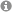The Role of sensory neuropeptides and catecholamines in fracture healing and fracture-stimulated autoimmune response
As a multi-step event, the endochondral ossification in the process of fracture healing represents an ideal model to combine the fields of rheumatic, immunologic and orthopaedic research due to that it involves processes like inflammation, angiogenesis and mesenchymal tissue repair. Previous studies have shown that nerve fibers of sensory and sympathetic origin innervate bone and fracture callus. These nerve fibers are located in the region of bone called Substancia compacta.
As the signals for proper callus tissue remodeling and successful endochondral ossification during the healing process are not known, we hypothesize that these nerve fibers play an important role. Experiments have shown that the absence of sensory innervations alters callus size and bone formation and may result in non-united fractures. Substance P (SP) is a sensory neuropeptide that influences cell proliferation, apoptosis, matrix formation and differentiation of mesenchymal callus tissue. Previous studies in our lab demonstrated that chondrocytes originating from callus tissue express SP and its receptor, the neurokinin 1 receptor (NK1-R). Earliest expression is detected at day 5 and prolongs at least until day 13.
&nb

Fig 1: Cartilage and bone formation during time course of fracture healing. Cartilaginous matrix is displayed in blue colour (alcian blue) and bony matrix is displayed in red colour (sirius red).

Fig. 2: Both, SP- and TH-positive nerve fibres seem not to penetrate the cartilage tissue as well as the newly formed woven bone whereas chondrocytes originated from callus tissue express SP and the neurokinin 1 receptor (NK1-R).
Our fracture model demonstrated characteristic stage-specific localizations of tyrosin– hydroxlyase (TH)- and substance P (SP)-positive fibres during the healing progress. Both, TH- and SP-positive nerves penetrate the callus in very early stages of the healing process, while at later time points when a cartilaginous matrix has been formed they retract towards the callus periphery.
TH-positive nerve fibres innervate blood vessels within the fracture callus, implicating a role in blood-flow regulation during callus differentiation. Furthermore the release of neural factors from the cartilage periphery may directly affect chondrocyte differentiation and matrix formation during callus maturation.
Chondrocytes originated from callus tissue express SP and its receptor (NK1-R). This implicates yet unknown, possible trophic functions of neuropeptides during cartilage differentiation and endochondral ossification in adults. Preliminary results of our chondrocyte pellet culture system suggest opposite effects of SP and NE on chondrocyte proliferation and matrix formation. Possibly, SP limits formation of a cartilaginous matrix and promotes formation of a bony matrix during endochondral ossification of callus in fracture healing.
The healing process leads to inflammatory reactions and exposure of extracellular matrix proteins to phagocytic cells and immune cells. If innervation of SP positive fibres dominate during the time course of endochondral ossification possibly due to a loss of sympathetic nerve fibers, the pro-inflammatory role of sensory nerve fibers could potentially stimulate an autoimmune response. In this context, SP might shift the balance to autoimmunity and lead to the development of autoimmune diseases as rheumatoid arthritis (RA).
Sympathetic neurotransmitters like norephinephrine (NE) might exert opposite effects on chondrocyte metabolism and matrix formation and possibly balance excessive and overshooting tissue repair processes. Therefore, we study the influence of SP and NE on murine chondrocytes in 2D-culture and a tibial explant culture model. Preliminary results of the 2D-chondrocyte culture model indicate that SP and NE have a dose-dependent effect on the expression of some marker genes concerning callus differentiation. In some cases they exert opposite effects as for mmp-3 but they also act in concert, e.g. timp-3.
&nb
Fig. 3: Effect of SP and NE on murine costal chondrocytes. Altered gene expression of cyclooxygenase (cox)-2 and matrix metalloproteinase 3 (mmp-3) after stimulation with IL-1β and SP or NE for 24h. Both, SP and NE repress gene expression of cox2 whereas NE induces expression of mmp-3 while SP reduces the mRNA level.
-->Grants
This work is supported by the BMBF as subproject in the consortium ImmunoPain (grant 01EC1004D)
Investigators
Susanne Grässel, Prof. Dr. rer. nat.; Tanja Niedermaier, Dipl. Biol.; Benjamin Craiovan Dr. med.; Anja Pasoldt, TA, Orthopaedic Surgery, University Hospital Regensburg
Collaboration:
Prof. Dr. Rainer Straub, Department of Internal Medicine I, University Hospital Regensburg
Dr. Richard Stange, Dr. Britta Wieskötter, Trauma Surgery, University Hospital Münster
Dr. Volker Kuhn, Clinic for Trauma Surgery and Sportsmedicine,Medical University Innsbruck, Austria
Prof. Georg Schaible, Dept. of Physiology 1, University Hospital Jena, Germany