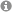Members of Regensburg Center Of Neuroscience
Thomas Baghai, Clinical Neurosciences
Prof. Dr. Thomas C. Baghai, Dept. of Psychiatry and Psychotherapy, University of Regensburg
+++under construction+++
Ulrich Bogdahn, Neurology
Prof. Dr. Ulrich Bogdahn, Klinik für Neurologie der Universität Regensburg am Bezirksklinikum
The group is concentrating on biomarker development and new treatments for neurodegenerative disorders, hereby focusing on ALS (amyotrophic lateral sclerosis). The group tries to use clinical available biomarkers from neurophysiology and neuroimaging as well as hematological parameters from stem cell biology to validate future biomarkers for clinical trials in this disease category. Also a new drug is being developed within the framework of the BMBF GO-Bio initiative; here the aim is clearly to stimulate compensatory neurogenesis in patients with neurodegenerative disorders. The compound being looked at is a cytokine signaling antagonist that will functionally restore neurogenic proliferation. The group is also a member of the German BMBF funded motoneuron network MND-Network coordinated by the University of Ulm. In addition we are also a member of the NISALS consortium, which tries to standardize neuro imaging procedures in ALS.
Intense cooperations with the Departments of Hematooncology, UKR; Institute of Neuropathology, UKR; Institute and clinics for Radiology and Neuroradiology, UKR/BKR; Institute of Microbiology, UKR
Oliver Bosch, Neurobiology and Animal Physiology
Prof. Dr. Oliver Bosch, Maternal Group, Lehrstuhl für Neurobiologie and Tierphysiologie, Universität Regensburg
Positive social relationships are beneficial for both physiological and psychological well-being. Here, the brain neuropeptides vasopressin and oxytocin as well as CRF and the urocortins have great impact. We study how these neuropeptides (dys-)regulate mother-pup bonding, the first bond mammals experience in their lifes. In addition, we are interested in the short- and long-term effects of breaking a male-female pair bond from the cellular to the behavioural level.
Alexander Brawanski, Neurosurgery
Prof. Dr. Alexander Brawanski, Klinik und Poliklinik für Neurochirungie, Universitätsklinikum Regensburg
Despite the improvement of surgical techniques, a number of diseases requiring neurosurgical management still show unsatisfying outcome. This is cardinally because the pathogenesis of these conditions is incompletely understood. Our research therefore focusses on the molecular basis of clinically relevant mechanisms in order to improve treatment outcome. We currently have segregated our projects in four main areas of research: 1. Subarachoid hemorrhage related vasospasm: The role of vasoactive peptides is evaluated using ELISA, Western blot and immunohistochemistry in correlation to clinical presentation and outcome (Fig. 1). 2. Neuro – Imaging: The main focus is on the preoperative segmentation of preoperative MRI imaging in patients with brain tumors. The ultimate goal is to define the most adequate surgical trajectory in order to achieve maximal tumor resection with minimal surgical toxicity (Fig.2). 3. Deep brain stimulation: This team employs advanced imaging modalities such as diffusion tensor imaging and tractography in order to optimize the position of deep brain stimulation electrodes (Fig.3). 4. Brain tumor biology: This more basic research oriented group focusses on the specific metabolism of malignant brain tumors with regard to pH regulation and cell survival (Fig.4). In addition, the mechanisms of bone invasion are analyzed using co-culture techniques, gene knockdown approaches and in vitro functional assays (Fig. 5).
Fig. 1: CT scan of a patient with subarachnoid hemorrhage; corresponding angiogram with severe vasospasm critically reducing the cerebral blood supply; immunofluorescence staining of Neuropeptide Y expressed by smooth muscle fibers of a brain micovessel (from: Molecular, cellular and developmental biology (www.yale.edu))
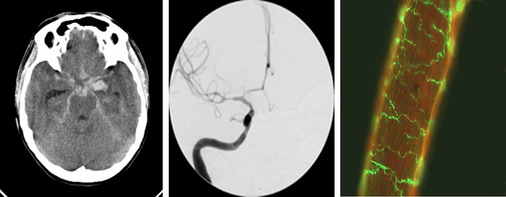
Fig. 2: Preoperative segmentation of a primary malignant brain tumor.

Fig. 3: Target planning for a deep brain stimulation procedure in a patient with Parkinson's disease. DTI imaging based fiber tracking highlights the internal capsule.

Fig. 4: Carboanhydrase IX expression in primary brain tumors increases with grade of malignancy

Fig. 5: Tumor – bone chip co-culture assay reveals extensive meningioma cell infiltration

Bjoern Brembs, Neurogenetics
Prof. Dr. Björn Brembs, Institute of Zoology – Neurogenetics
In our lab, we study how brains generate spontaneous behavior, how they evaluate the sensory feedback from these actions and how different states of the animal influence the way these processes take place. Our various research projects are motivated by the general insight that brains are active organs: rather than passively responding to external stimuli, brains actively initiate behaviors to probe the environment and then evaluate its response in order to gain the maximum amount of control. Because we believe these to be very fundamental aspects of general brain function, common to all brains, we study the nervous system of invertebrates, mainly the fruit fly Drosophila. We test wild type, mutant and transgenic flies in various behavioral paradigms for deficits in spontaneous behavior, operant learning, attention-like processes, decision-making, motivation or motor control.
Bjoern.Brembs@biologie.uni-regensburg.de
Barbara Di Benedetto, Neuro-glial Pharmacology
Dr. Barbara Di Benedetto, AG Neuro-gliale pharmakologie, Lehrstuhl für Psychiatrie und Psychotherapie, Universität Regensburg
Astrocytes connect synaptic and vascular brain compartments in the so-called neurovascular unit (NVU). They regulate both development and functions of neuronal networks (ie synapses) and blood-brain barrier. Therefore, if their functionality is altered by either genetic predisposition or exposition to adverse environmental conditions, astrocytes might inefficiently exert their roles with consequent onset of neuropsychiatric disorders. The lab focuses on understanding the role of astrocytes in the etiopathogenesis of depressive and anxiety disorders and which astrocytic properties are targeted by pharmacological treatments to rescue disease phenotypes. Applying histologic, molecular and biochemical methods to cell cultures and brain tissue derived from either wildtype rodents or animal models of depression/anxiety, we aim at examining which astrocyte-specific genetic and epigenetic variations might represent predisposing factors to develop neuropsychiatric disorders and how pharmacological treatments might modulate such variations to restore brain homeostasis
Stefan Dove, Pharmaceutical and Medicinal Chemistry
Prof. Dr. Stefan Dove, Lehrstuhl Pharmazeutische/Medizinische Chemie II
G-protein coupled receptors (GPCRs) are the most important targets in the pharmacological therapy of CNS disorders. Computer-assisted methods (structure-based design, quantitative structure-activity relationships) are significantly involved in the development and optimization of CNS drugs. In the meanwhile, crystal structures of more than 20 GPCRs have been resolved. Homology models of other GPCRs and of complexes with G-proteins, docking of agonists and antagonists as well as MD simulations contribute to the investigation of binding modes, of molecular reasons for ligand selectivity and of mechanisms of GPCR-based signal transduction. With regard to neuropharmacology, we recently modeled interactions of 5-HT2A receptors with partial agonists and of histamine receptor subtypes with tricyclic antidepressants and neuroleptics.
stefan.dove@chemie.uni-regensburg.de
-->Gesine Dreisbach, General and Applied Psychology
Prof. Dr. Gesine Dreisbach, Chair for General and Applied Psychology, University of Regensburg
Our lab focuses on processes of cognitive control. Cognitive control allows humans to dynamically adjust attention, thought, and action to changing goals and task demands. Currently, we pursuit four lines of research:
o Context-sensitive adjustment of cognitive control
o Response conflicts as aversive signals for control adaptations
o Affective and motivational modulation of cognitive control
o Task switching as a tool to study cognitive flexibility
• Research methods: Behavioral studies, reaction times, error rates, peripher-physiological markers of affect and arousal, EEG and event-related potentials (under construction)
gesine.dreisbach@psychologie.uni-regensburg.de
-->Veronica Egger, Neurophysiology
Prof. Dr. Veronica Egger, AG Neurophysiology (group), Chair for Neurobiology and Animal Physiology, University of Regensburg
Coding of olfactory stimuli is still not fully resolved and involves special types of neuronal microcircuits that otherwise are found mainly in other sensory-related brain areas, namely in the retina and thalamus. We aim to understand the function of these microcircuits at several levels. Presently, we study neuronal processing in the olfactory bulb at single synaptic resolution using two-photon Ca2+ imaging and two-photon uncaging in combination with whole cell electrophysiological recordings. We also use population imaging and field potential recordings to investigate network function and are on the verge of carrying these techniques to a more systemic level.
Veronica.Egger@biologie.uni-regensburg.de
Peter Flor, Molecular and Cellular Neurobiology
Prof. Dr. rer. nat. Peter Flor, Molecular and Cellular Neurobiology and Neuropharmacology, University of Regensburg
Research in our laboratory is focused on trying to better understand the complex interactions, at a molecular and cellular level, between stress and genetics that lead to the manifestation of multiple pathologies such as anxiety, reactive depression, neuronal loss, substance abuse and cognitive dysfunction in certain susceptible individuals.
This effort is paralleled with investigations towards discovery and characterization of new pharmacological therapies – eventually aiming to counteract the deleterious consequences of acute and chronic stress. To this end, we currently focus on pharmacological modulation of metabotropic glutamate receptor (mGlu) subtypes, which are known to play key roles within the emotion circuitry of the brain, e.g. mGlu5 and mGlu7.
Peter.Flor@biologie.uni-regensburg.de
Mark Greenlee, Experimental Psychology
Prof. Dr. rer. nat. Mark Greenlee, Chair of Experimental Psychology, University of Regensburg
Our group focuses on research topics in the area of human cognitive neuroscience, sensory processing and experimental neuropsychology. We use functional and structural MRI, EEG, eyetracking and psychophysics to address these issues. Collaborations with several international groups are currently on-going. In particular our research program address the following topics:
o cortical mechanisms underlying multi-sensory processing (i.e., audio-visual, visual-vestibular, visual-tactile) with an emphasis on self-motion perception and spatial perception;
o neural plasticity as a consequence of retinal or cortical lesions;
o brain changes following training and perceptual learning;
o perceptual decision making and planning;
o saccadic and pursuit eye movements during visual search;
o language processing in healthy controls and in the patients (pre-surgical mapping);
o as well as methodological issues.
Mark.Greenlee@psychologie.uni-regensburg.de
Thilo Hinterberger, Angewandte Bewusstseinswissenschaften
Prof. Dr. Thilo Hinterberger, Forschungsbereich Angewandte Bewusstseinswissenschaften, Abteilung für Psychosomatische Medizin, Universitätsklinikum Regensburg
Der Forschungsbereich Angewandte Bewusstseinswissenschaften der Abteilung für Psychosomatische Medizin befasst sich mit folgenden Themen:
- der Analyse neuropsychologischer Korrelate von Bewusstseinsmodellen und Bewusstseinszuständen, wie sin in Meditation, Hypnose, aber auch verschiedenen Pathologien auftreten
- Erfahrung mit den Methoden EEG (ereigniskorreliert Potenziale, Mapping, …), ECoG, fMRI, EKG (HRV), EDA,…
- Entwicklung und Evaluation neuartiger Neurofeedbackmethoden, z.B. das Sensorium, eine Neurofeedbackumgebung zur erweiterten Selbstwahrnehmung
- Kompetenz in der Entwicklung und Anwendung von Brain-Computer Interfaces
- Evaluation von Verfahren in Psychosomatischen Kliniken
Weitere Informationen unter www.ab-wissenschaften.de
Stichworte:
Neuropsychologie, veränderte Bewusstseinszustände, Meditation, Neurofeedback
Methoden:
EEG (64-Kanal), ereigniskorreliert Potenziale, Elektrophysiologie, Neurofeedback, Brain-Computer Interfaces, Softwareentwicklung, Echtzeitanalyse
Kontakt:
Tel.: +49 941 944 2748
E-mail: Thilo.Hinterberger@ukr.de
Eugen Kerkhoff, Molecular Cell Biology
Prof. Dr. Eugen Kerkhoff, Departmentent of Neurology, Molecular Cell Biology Laboratory, University Hospital Regensburg
My lab focuses on the regulation of vesicle transport processes by actin filaments and associated myosin motor proteins. We study the functional cooperation of the Spir/formin actin nucleator complex, which initiates the polymerization of actin filaments at vesicle membranes, and moysin V actin motor proteins. The mammalian myosin Va and Vb motor proteins are effectors of the Rab11 small G-protein and function in dendrite branching and post-synaptic AMPA receptor recycling. Mouse genetics show an implication of Spir and formin-2 function in emotional learning and memory. Mutation of the human myosin Va protein (Griscelli syndrome type 1) causes severe primary neurologic defects including mental retardation. Human genetics further indicate that alterations in myosin Vb function could cause schizophrenia. We determine the function and protein complex dynamics of the Spir/formin/MyoVb/Rab11 proteins by live cell imaging methods (TIRF, epi-fluorescence, FLIM-FRRET, FRAP and FCCS) and in vitro with purified proteins and biomimetic membrane systems. The studies are performed in a DFG funded collaboration with Petra Schwille and Thomas Weidemann (MPI of Biochemistry, Martinsried).
Eugen.Kerkhoff@klinik.uni-regensburg.de
-->Brigitte Kudielka, Medical Psychology
Prof. Dr. Brigitte Kudielka, Chair of Medical Psychology, Psychological Diagnostics and Research Methodology, University of Regensburg
Main research topics:
o Stress research – Identification and description of psychological and biological determinants of individual stress regulation
Research aims:
o -To identify mechanisms explaining how stress-related health disturbances develop and to identify psychological and biological factors of inter- and intraindividual stress vulnerability
o -Integration of psychobiological and work psychological research methods to assess differential stress patterns under chronic work stress
o The overarching research topic is the phenomenon of stress. Stress and stress-related diseases are major problems of our modern and industrialized societies and, not least, cause enormous economic costs. Knowledge on psychological and biological mechanisms how stress increases the risk for the development or progression of a manifest disease, however, is still fragmentary. Therefore, our aim is to identify relevant psychobiological mechanisms of stress regulation in an interdisciplinary and multi-methodological approach. Beside others, we are interested in research questions like the following: Which psychic, neuronal, endocrine and immunological processes play an important role in the development of stress-related diseases? Why do some people develop an illness or disease under conditions of chronic stress while others don’t? Which factors influence the individual stress vulnerability and stress resilience? Do, for example, factors like gender, age, personality traits or situational characteristics like the psychosocial work environment contribute significantly? Major research topics are thus the phenomenon of stress, the subjective experience of stress, psychobiological aspects of stress, health consequences of stress, and diagnostic aspects. Finally, a special focus lies on the issue of stress at work.
Brigitte.Kudielka@psychologie.uni-regensburg.de
Berthold Langguth, Brain Stimulation and Neuromodulation
Prof. Dr. Berthold Langguth, Dept. of Psychiatry and Psychotherapy, University of Regensburg
Neuroplasticity reflects the ability of the central nervous system to learn and to adapt to a changing environment. However, under certain circumstances neuroplastic mechanisms can become maladaptive and cause neurological and psychiatric symptoms and syndromes. The goal of the research group is the identification of maladaptive changes in neuro-psychiatric disorders and their modification by brain stimulation and neuromodulatory approaches.
For the identification of abnormal neuroplastic processes neuroimaging (functional and structural Magnetic Resonance Imaging, Near Infrared Spectroscopy) and electrophysiological methods (Electroencephalography, evoked potentials) are used. For Neuromodulation brain stimulation techniques such as transcranial magnetic stimulation (TMS), transcranial electrical stimulation (tES) or deep brain stimulation as well as pharmacotherapy, specific forms of psychotherapy, neurobiofeedback or auditory stimulation are investigated.
Clinical research areas include affective disorders, schizophrenia, tinnitus, sleep and sexual disorders, but also preclinical studies in healthy controls are performed.
Seth Levine, Biomedical Imaging
Seth Levine, PhD, Biomedical Imaging Unit, Department of Psychiatry and Psychotherapy, University of Regensburg
Juan M. Lima-Ojeda, Clinical Neurosciences
Dr. Juan M. Lima-Ojeda, Dept. of Psychiatry and Psychotherapy, Clinical Neurosciences, University of Regensburg
André Manook, Clinical Neurosciences
Dr. med. André Manook, Dept. of Psychiatry and Psychotherapy, Clinical Neurosciences, University of Regensburg
Andreas Mühlberger, Clinical Psychology and Psychotherapy
Prof. Dr. Andreas Mühlberger, Clinical Psychology and Psychotherapy, Dept. of Psychology, University of Regensburg
We are interested in the etiology and the course of mental disorders as well as the processes that lead to effective treatments for these disorders. Our main focus is on anxiety disorders. Further research-relevant disorders are addiction, affective disorders, and pain-related disorders. Methods used are mainly experimental studies and the assessment of self reports, behavioral (e.g., approach behavior), and physiological (peripheral as heart rate, skin conductance and startle responses and central as EEG and fMRI) parameters that reflect affective learning. As a highly controllable and ecological valid research tool we often use Virtual Reality to establish experimental paradigms and learning environments. In our basic science branch we apply conditioning procedures to investigate genetic and neural aspects of fear and anxiety that might be relevant for anxiety disorders. In our applied research branch we evaluate neural modulators and outcome of psychological treatments using our outpatients department.
Andreas.Muehlberger@psychologie.uni-regensburg.de
-->Inga Neumann, Neurobiology and Animal Physiology
Prof. Dr. Inga Neumann, Lehrstuhl für Neurobiologie und Tierphysiologie, Universität Regensburg
Brain neuropeptides such as oxytocin, vasopressin, CRF and neuropeptide S are major modulators of various emotional (anxiety- and depression-related behaviours) and social (social interaction, preference and memory, inter-male and inter-female aggression, reproductive behaviours) behaviours. We aim to reveal the molecular and neuronal mechanisms underlying these effects of neuropeptides on social and emotional behaviours under physiological and pathophysiological conditions. Using molecular-genetic and neuroendocrine techniques in combination with adequate animal models and behavioural tests we specifically study the receptor-mediated intraneuronal signaling cascades, and epigenetic and genetic mechanisms underlying these neuropeptidergic functions.
Inga.Neumann@biologie.uni-regensburg.de
Caroline Nothdurfter, Molecular Psychopharmacology
PD Dr. Caroline Nothdurfter, Dept. of Psychiatry and Psychotherapy, University of Regensburg
The translocator protein 18 kDa (TSPO) is a mitochondrial protein, which is important for neurosteroid synthesis and systemic endocrine regulation, with implications for the pathophysiology of immune, inflammatory, neurodegenerative, neoplastic as well as psychiatric diseases. Some TSPO ligands already play a role in the pharmacological treatment of anxiety disorders. Our lab aims to collect evidence whether TSPO ligands may also be useful in the treatment of depression and other stress-related disorders by using a translational approach including cell culture models and clinical studies.
Markus Riemenschneider, Neuropathology
Prof. Dr. Markus J. Riemenschneider, Abteilung für Neuropathologie, Universitätsklinikum Regensburg
Our lab focuses on the investigation of the molecular genetic basis of human brain tumors (gliomas). These tumors harbor an intrinsic ability to malignant progression and they tend to diffusely infiltrate the surrounding brain tissue, thus limiting the efficiacy of the currently available therapeutic approaches. Consequently, an improved understanding of the molecular genetic aberrations and signaling pathways underlying glioma cell invasion and glial tumor progression appears of considerable importance for the development of more efficient and targeted tumor therapies. To achieve this goal, our laboratory emphasizes the combination of in vitro-techniques with histology-based approaches that allow for the direct study of molecular changes in human glioma tissue specimens. Our translational research approach enables us to convey basic research results into clinical neuropathological applications with the aim of identifying novel diagnostic and prognostic biomarkers. A main methodical focus is on the identification and functional characterization of genes that are epigenetically regulated, either by means of promoter methylation, aberrant histone modification patterns or a regulatory network of small, non-coding RNAs (so-called miRNAs). In doing so we use a broad spectrum of techniques that involve molecular biology approaches, cell-based assays and array / next generation sequencing applications. Since 2013 our lab also participates in the Bavarian Research Network on induced pluripotent stem cells (ForIPS) funded by the Bavarian State Ministry of Education, Science and the Arts. Here, we extend our methodical repertoire to the investigation of the epigenetic stability of human induced pluripotent stem cells and to the molecular basis of Parkinson’s disease.
Markus.Riemenschneider@klinik.uni-regensburg.de
Rainer Rupprecht, Psychiatry and Psychotherapy
Prof. Dr. Rainer Rupprecht, Lehrstuhl für Psychiatrie und Psychotherapie, Universität Regensburg
Ärztlicher Direktor der Klinik und Poliklinik für Psychiatrie und Psychotherapie der Universität Regensburg am Bezirksklinikum Regensburg
Our group runs a translational research program dedicated to affective disorders, especially depression and anxiety. Within the BMBF “German Research Network for Psychiatric Disorders” our group is coordinating the research network “Optimized Treatment of Depression“ (OptiMD) with seven projects at eight universities/institutions. Our research profile comprises basic on the molecular pharmacology of antidepressants and is translated into animal studies using appropriate disease models. Moreover, we are interested in the pharmacology and physiology of neurosteroidogenesis, e.g. via the translocator protein 18 kDa (TSPO). Human proof-of-concept studies, which also are extended to neuroimaging, bridge the gap to clinical and endophenotypical characterization of patients with affective disorders.
Lehrstuhl für Psychiatrie und Psychotherapy der Universität Regensburg
Klinik und Poliklinik für Psychiatrie und Psychotherpie der Universität Regensburg am Bezirksklinikum Regensburg
-->Stephan Schneuwly, Developmental Biology
Prof. Dr. Stephan Schneuwly, Lehrstuhl für Entwicklungsbiologie, Institut für Zoologie, Universität Regensburg
Using Drosophila melanogaster as a model system, we are focusing our research on the development and stability of the fly nervous system. We take advantage of the powerful genetic, developmental, and behavioral methods established in Drosophila to study signaling pathways affecting the determination and differentiation of neurons from embryogenesis up to the adult stage. In addition we have developed several new animal models for neurodegenerative diseases like Parkinson and Friedreichs ataxia. Using these genetic models we are analyzing important cellular and molecular mechanisms leading to age dependent degeneration of the nervous system deciphering signaling pathways involved in this process.
stephan.schneuwly@biologie.uni-regensburg.de
-->Jens Schwarzbach, Biomedical Imaging
Prof. Dr. Jens Volkmar Schwarzbach, Biomedizinische Bildgebung, Lehrstuhl für Psychiatrie und Psychotherapie, Universität Regensburg
...under construction...
-->Bernhard Weber, Human Genetics
Prof. Dr. rer. nat. Bernhard Weber, Institute of Human Genetics, University of Regensburg
Our research focuses on degenerative processes of the retina and its support structures. In particular, we are interested in understanding disease pathologies of the hereditary retinal degenerations and the genetically complex age-related macular degeneration (AMD). Ongoing research involves the identification of the genetic factors associated with retinal disease, the development and analysis of animal models as tools to understand the in vivo function and dysfunction of retinal disease genes. Importantly, our research which addresses genetic variation and its consequences on cellular functions has been greatly extended by including state-of-the-art technologies such as induced pluripotent stem cell approaches to generate cellular model system for patient-derived retinal and retinal pigment epithelium cells. This work has a strong focus on translational aspects to explore concepts of innovative therapeutic intervention in retinal disease. In this context, the Institute of Human Genetics has a longstanding commitment towards DNA diagnostics for hereditary retinal dystrophies. Our DNA diagnostics department offers single gene analyses and gene panel tests by using next generation sequencing to address the striking genetic heterogeneity in retinitis pigmentosa, cone rod dystrophy and other retinal disorders.
Some reading:
https://www.ncbi.nlm.nih.gov/pubmed/25203061
https://www.ncbi.nlm.nih.gov/pubmed/24801942
https://www.ncbi.nlm.nih.gov/pubmed/24622760
https://www.ncbi.nlm.nih.gov/pubmed/22666427
https://www.ncbi.nlm.nih.gov/pubmed/22245536
Bernhard.Weber@klinik.uni-regensburg.de
Robert Weissert, Clinical Neurobiology
Prof. Robert Weissert MD PhD, Clinical Neurobiology, Dept. of Neurology, University of Regensburg
My group is focusing on elucidating the encephalitogenic immune response in multiple sclerosis (MS) patients (Weissert, 2013). Peptides presented on major histocompatibility complex (MHC) I and II molecules in the central nervous system (CNS) are possibly of paramount importance in orchestrating the immune response that leads to lesion development in MS. We have eluted ligands of MHC I and II of the CNS of patients with MS (Fissolo et al. 2009). We are presently assessing the immune response to such peptides of T cells from blood of MS patients. We have collected peripheral blood mononuclear cells (PBMC) of over 200 patients with different types of MS (Riedhammer et al., 2014). Moreover we are interested in assessment of cognition in patients with MS. Also we would like to contribute to a better understanding of emergence of cortical lesions in patients with MS (Storch et al., 2006). Possibly novel autoantigens are involved in grey matter pathology of MS that we would like to describe by state of art methodologies/technologies. Autoantigen structure and modification are possibly of paramount importance in driving disease development in MS (de Graaf et al. 2012).
- de Graaf KL, Albert M, Weissert R. Autoantigen conformation influences both B- and T-cell responses and encephalitogenicity. J Biol Chem. 2012, 287:17206-17213.
- Fissolo N, Haag S, de Graaf KL, Drews O, Stevanovic S, Rammensee HG, Weissert R.Naturally presented peptides on major histocompatibility complex I and II molecules eluted from central nervous system of multiple sclerosis patients. Mol Cell Proteomics. 2009, 8:2090-2101.
- Riedhammer C, Halbritter D, Weissert R. Peripheral Blood Mononuclear Cells: Isolation, Freezing, Thawing, and Culture. Methods Mol Biol. 2014 [Epub ahead of print]
- Storch MK, Bauer J, Linington C, Olsson T, Weissert R, Lassmann H. Cortical demyelination can be modeled in specific rat models of autoimmune encephalomyelitis and is major histocompatibility complex (MHC) haplotype-related. J Neuropathol Exp Neurol. 2006, 65:1137-1142.
- Weissert R. The immune pathogenesis of multiple sclerosis. J Neuroimmune Pharmacol. 2013, 8:857-866.
Thomas Wetter, Clinical Sleep Research
Prof. Dr. Thomas Wetter, Clinical Sleep Research, Dept. of Psychiatry and Psychotherapy, University of Regensburg
Sleep research has a long tradition at the psychiatric clinic and it continues to be a research topic that is closely inter-linked with other project lines, in particular depression. Clinical sleep research focuses on primary and comorbid insomnia, daytime sleepiness including narcolepsy, parasomnias, and sleep-related movement disorders such as the restless legs syndrome.
There is a strong link between sleep and psychiatric disorders. Sleep of adequate duration and quality is a prerequisite for daytime functioning but disturbed sleep is a hallmark of psychiatric disorders, in particular affective disorders. In addition to the rather unspecific impaired sleep macrostructure, microstructuralelements such as the signature of sleep EEG or the amount of rapid eye movements (REMs) during sleep emerged as distinguishing and potentially predictive features in patients with affective disorders. Importantly, most antidepressants exert pronounced effects on sleep EEG.
In addition, our research aims to elucidate neurobiological mechanisms of specific sleep disorders such as narcolepsy and restless legs syndrome. We participate in treatment studies with a focus on primary insomnia and daytime sleepiness, using both cognitive behavioural techniques and new medications.
Christian Wetzel, Molecular Neurosciences
Prof. Dr. Christian Wetzel, Molecular Neurosciences, Dept. of Psychiatry and Psychotherapy, University of Regensburg
We are interested in the molecular aetiology, pathology and pathogeneses of psychiatric disorders. By means of electrophysiological and live cell imaging approaches, we analyse inter- and intracellular signalling mechanisms in order to understand the role of mitochondrial dysfunction in the aetiology of depression and anxiety disorders.
A second project deals with the functional characterisation and structure/function analysis of transient receptor potential (TRP) ion channels. TRPV1 channels are of particular interest, since they are involved in nociception as well as in pathophysiological pain and modulate synaptic transmission in certain areas of the brain.