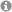Nanoparticle Distribution in Tissues and Organs

Nanoparticle distribution in the posterior segment of the eye. Nanoparticles accumulate in the endothelial cells of the choriocapillaris (CC). Sclera (S); retinal pigment epithelium (RPE). Fluorescent nanoparticles (red); DAPI nuclei staining (blue); tissue autofluorescence (gray). (Figure taken from: Pollinger et al. Proc. Natl. Acad. Sci. 2013, 110, 6115–6120, www.pnas.org/content/110/15/6115).
Nanoparticles are subject to limited biodistribution due to their size and physicochemical properties which is a severe handicap for their therapeutic application. An exception is tumor tissue into which nanoparticles may extravasate through endothelial fenestrations. These openings stem from changes in malignant tissue blood vessels, particularly in capillaries. But endothelial fenestrations also exist in the capillaries of healthy tissues such as the kidneys’ glomeruli and the choroid. In both cases, the therapy of several diseases would benefit tremendously from the capability of therapeutic nanoparticles to specifically leave the bloodstream and enter adjacent tissue. Target of our work is to comprehend which characteristics of nanoparticles enable them to reach non-malignant tissues after their parenteral application.
Multivalently and Heteromultivalently Binding Nanoparticles

Sagittal eye sections of mice that received 200 pmol either ligand-decorated (right figure) or or ligand-free quantum dot (Qdot) nanoparticles (right figure). Upon systemic administration only EXP3174-modified Qdots accumulated in the choriocapillaris and in intraretinal capillaries of the posterior eye. (CC: choriocapillaris, RPE: retinal pigment epithelium, OS: outer segment, IS: inner segments, ONL: outer nuclear layer, OPL: outer plexiform layer, INL: inner nuclear layer, fluorescent nanoparticles (red); DAPI nuclei staining (blue); tissue autofluorescence (gray). (Figure taken from: Hennig et al., Multivalent nanoparticles bind the retinal and choroidal vasculature. Journal of controlled release 220 (2015) 265–274. DOI: 10.1016/j.jconrel.2015.10.033)
A frequently discussed topic in the field of biomaterials and their use for targeted drug delivery is the development of tailored nanoparticles for highly specific interactions with cell surface receptors.
This so-called direct targeting is based on receptor-specific ligands that are immobilized in the nanoparticle corona and that are intended to enhance the particle’s avidity for the target cell.
However, both the ligands’ attachment to particle surfaces as well as their simultaneous presentation during cell-particle interaction are particularly different from a classical ligand receptor interaction.
On the one hand, ligands tend to lose part of their capability to bind the target receptor with high affinity due to their attachment to the material surface. On the other hand, ligand-modified nanoparticles can simultaneously bind multiple receptors in a coordinated manner and thereby overcome the individual ligand’s affinity loss, resulting in a possibly enhanced overall avidity. Goal of our work is to study the underlying mechanisms of this multivalent binding and try to carve out the overall avidity of the nanoparticle-cell interaction. A further aspect of our studies focuses on so-called off-target cells, i.e. cells that potentially carry the same receptor as the target cells and thereby lower the target accuracy of our nanoparticles. Viruses as an example of “natural” nanoparticles can evade this hazard by concomitantly binding to a set of several receptors to identify their target. We transfer this principle to nanoparticles in a biomimetic approach and increase thereby their target cell specificity. Our approach relies on nanoparticles carrying specific ligands for different receptors. These ligands are expected to not bind simultaneously but sequentially, as it is the case for viruses.
Pharmaceutical Technology
Nanoparticle Distribution in Tissues and Organs
Interactions of materials with cells and tissues
Drug Delivery