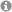Tin-bismuthides
By substituting individual corners of the homoatomic clusters with heteroatoms that differ in size, electronegativity or valence electron number, the reactivities of the Zintl ions can be directly influenced.
Synthesis heteroatomic Zintl anions
Heteroatomic Zintl anions can be obtained by the extraction of mixed-valent solid phases or by the direct reduction of the elements in liquid ammonia. In this way a number of different tin-bismuthides can be obtained. The low temperatures of the liquid ammonia contribute significantly to the stabilization of (energetically) metastable anions such as [Sn₃Bi₃]⁵⁻.

Fig.1: Synthesis of different tin-bismuthides.
Functionalization of heteroatomic Zintl anoins
As with homoatomic clusters, research is also being carried out into the functionalization of heteroatomic cluster anions. The focus here is on the use of transition metal complexes such as metal carbonyls and NHC complexes.