Research
Structural and functional biology of proteins
Signal transduction protein PII and its interacting partners
We are interested in studying PII and PII-like proteins that belong to a family of trimeric cytoplasmic signaling proteins and are found in wide variety of organisms. It is conserved among bacteria, archaea and plants. They are thought to involve in the carbon / nitrogen balance and also act as a sensor in these organisms. They are one of the largest and most ancient families of signaling proteins. We are also interested in the exploring PII proteins from different organisms and its interacting partners.
Free electron laser and methodology development
Multiheme cytochromes c (MCCs) from Ignicoccus hospitalis
Multiheme cytochromes c (MCCs) constitute an important class of proteins that have received considerable attention in recent decades because of their central role in global nitrogen, sulfur and iron cycling and its impact on the climate system. The poorly characterized clade II of the octaheme cytochromes c (OCC) family is closely related to both the hydroxylamine oxidoreductase (HAO) and octaheme nitrite reductase (ONR) families. we have detailed functional and structural studies of the highly abundant IhOCC (Ignicoccus hospitalis octaheme cytochrome), including an X-ray crystal structure at 1.7 Å resolution, the first three-dimensional structure of an archaeal OCC (Figure 1). The trimeric IhOCC is membrane associated and exhibits significant structural and functional differences to previously characterized homologs within the hydroxylamine oxidoreductases (HAOs) and octaheme cytochrome c nitrite reductases (ONRs).
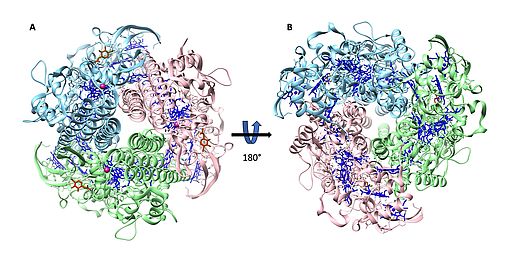
Figure 1
Three-dimensional structure of IhOCC from Ignicoccus hospitalis. Each subunit is shown in a different color (pink, blue, green). The (A) top and (B) bottom views of IhOCC. Hemes represented in stick mode are colored blue, Ca2+ ion (dark pink) and N-acetylglucosamine (NAG) in red respectively.